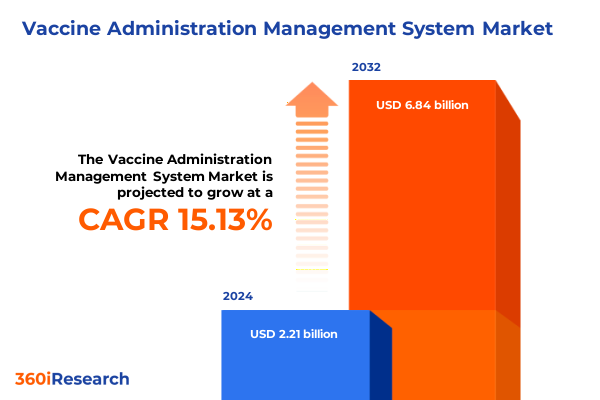
The Vaccine Administration Management System Market size was estimated at USD 329.40 million in 2025 and expected to reach USD 367.32 million in 2026, at a CAGR of 11.01% to reach USD 684.33 million by 2032.
Accelerating Vaccination Efficiency Through Integrated Administration Management Systems Driving Public Health Outcomes and Operational Excellence
The escalating complexity of global immunization programs demands a holistic solution capable of streamlining workflows, strengthening compliance, and delivering real-time visibility into vaccine administration operations. In response, integrated vaccine administration management systems have emerged as pivotal enablers for healthcare providers, public health authorities, and research institutions seeking to optimize end-to-end processes. By uniting scheduling, inventory control, reporting analytics, and communication channels, these platforms are transcending traditional silos to facilitate swift decision making and reduce administrative burden.
As health organizations confront challenges ranging from cold chain integrity to patient engagement, a centralized management approach is rapidly gaining traction. This executive summary examines the transformative shifts reshaping the vaccine administration management landscape, analyzes the cumulative repercussions of United States tariff adjustments in 2025, highlights critical market segmentation insights, and distills key regional and competitive dynamics. The findings culminate in strategic recommendations tailored to guide industry leaders toward sustainable growth and operational excellence.
Emerging Technological and Operational Paradigm Shifts Redefining Vaccine Distribution and Administration Management Workflows Across Healthcare Ecosystems
The vaccine administration management sector is undergoing a revolutionary transformation driven by advances in digital health technology and heightened regulatory scrutiny. Cloud-native architectures, combined with robust data analytics engines, are converging to deliver predictive insights that preempt supply disruptions and forecast appointment demand with unprecedented accuracy. Concurrently, the integration of Internet of Things (IoT) sensors within refrigeration units is empowering stakeholders with continuous visibility into temperature excursions, thereby safeguarding vaccine efficacy.
Moreover, interoperability standards such as FHIR (Fast Healthcare Interoperability Resources) are breaking down barriers between electronic health records and inventory databases, enabling seamless data exchange across heterogeneous systems. This shift not only accelerates front-line workflows but also fortifies reporting capabilities for public health agencies. As a result, organizations that embrace these technological innovations while maintaining strict compliance with evolving regulations are positioned to achieve significant gains in patient satisfaction, resource utilization, and overall program resilience.
Assessing the Comprehensive Impacts of the 2025 United States Tariff Measures on Vaccine Administration Management Supply Chains and Technology Procurement
In 2025, the United States implemented new tariff measures on key components used in vaccine administration management hardware, including networking devices, refrigeration systems, and printers. These levies have introduced upward pressure on procurement costs for on-premise deployments, prompting many end users to reevaluate their capital expenditure strategies. Healthcare providers are now balancing the increased cost of rugged scanners and specialized refrigeration units against the benefits of long-term asset ownership.
As tariffs drive hardware expenses higher, service-oriented consumption models-encompassing managed hybrid and self-managed hybrid deployments-are gaining momentum. Organizations are shifting toward subscription-based licensing for compliance, tracking, and analytics software to mitigate capital outlays. Simultaneously, domestic manufacturing partnerships are being explored to localize critical supply chains and shield operations from further tariff volatility. Together, these adjustments underscore a broader industry drive toward cost efficiency, supply chain resilience, and adaptive deployment configurations.
Illuminating Critical Market Segmentation Dynamics Across End Users Components Deployment Modes and Application Use Cases Inform Strategic Positioning
Market segmentation insights reveal the diverse requirements and procurement behaviors across end users and the components underpinning administration management systems. Government agencies and public health centers, spanning local public health clinics and state departments, prioritize robust reporting and analytics capabilities that support large-scale immunization initiatives. By contrast, hospitals and clinics-encompassing community clinics, private hospitals, and publicly funded hospitals-seek comprehensive scheduling and appointment management modules with features such as automated reminders, mobile scheduling, and online booking portals that drive patient uptake.
Pharmacies, both chain and independent operations, value inventory management and forecasting solutions that minimize wastage and optimize stock rotation, while research and academic institutions require secure compliance and tracking software to support clinical studies and vaccine trials. On the component front, end users differentiate between hardware investments in networking devices, refrigeration systems, and barcode scanners, and the necessity for consulting, implementation, and ongoing training services. Software segments gravitate toward modular suites covering compliance tracking, demand forecasting, inventory control, advanced reporting analytics, and scheduling functionalities.
Deployment modes further shape purchasing decisions; cloud-based environments-offered in both public and private cloud configurations-drive rapid scalability for programs with seasonal immunization peaks, whereas hybrid architectures, whether self-managed or delivered as a managed service, strike a balance between data sovereignty and operational agility. On-premise installations leveraging dedicated or virtualized servers remain prevalent among organizations with strict data residency and security mandates. Finally, application segmentation demonstrates the necessity to tailor platforms for adult immunization campaigns, specialized occupational immunization of healthcare workers and military personnel, pediatric vaccination schedules, and travel immunization services designed for both business and personal itineraries. These layered segmentation perspectives inform strategic positioning and product roadmaps across the market.
This comprehensive research report categorizes the Vaccine Administration Management System market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- End User
- Component
- Deployment Mode
- Application
Unveiling Differential Regional Adoption Trends and Growth Drivers for Vaccine Administration Management Systems Across Global Markets
Regional landscapes exhibit distinct adoption curves and growth drivers for vaccine administration management solutions. In the Americas, established healthcare infrastructures combined with ongoing public health funding fuel digital transformation initiatives, particularly within large academic centers and government-led immunization campaigns. North American providers are increasingly investing in integrated platforms that offer end-to-end traceability, while Latin American markets are prioritizing cloud-based deployments to overcome legacy system constraints and accelerate program rollout.
Europe, the Middle East, and Africa present a tapestry of regulatory requirements and resource availability. Western European nations capitalize on advanced interoperability frameworks to align national immunization registries with hospital and pharmacy networks, whereas emerging markets in Eastern Europe and the Middle East are focusing on capacity building through managed services and training programs. In Africa, limited infrastructure has accelerated adoption of mobile scheduling and remote monitoring services that operate over low-bandwidth networks, ensuring critical vaccine reach even in rural areas.
Asia-Pacific demonstrates a bifurcation between highly sophisticated markets in Japan, South Korea, and Australia-where predictive analytics and AI-driven forecasting are mainstream-and rapidly developing economies in Southeast Asia and South Asia, which lean toward hybrid and cloud-first strategies to minimize initial capital outlay. Cross-border collaborations and regional consortia are fostering shared best practices, further reinforcing a dynamic environment where adaptability and local customization are key to successful implementations.
This comprehensive research report examines key regions that drive the evolution of the Vaccine Administration Management System market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Innovators Highlighting Strategic Partnerships Competitive Differentiators Defining the Vaccine Administration Management Landscape
Leading participants in the vaccine administration management sphere are distinguishing themselves through strategic partnerships, targeted acquisitions, and continuous innovation across hardware, software, and service domains. Established electronic health record vendors have integrated scheduling, inventory, and compliance modules to extend their care coordination suites, while specialized software firms are focusing on advanced analytics and mobile-first interfaces to meet demand for user-centric scheduling solutions with automated reminders and mobile access.
Hardware manufacturers are responding to tariff-induced cost pressures by developing modular refrigeration systems with remote monitoring capabilities that optimize energy consumption and minimize spoilage risk. Concurrently, service providers are expanding their consulting and implementation practices to support hybrid deployment models, offering phased rollouts and customizable training programs that accelerate user adoption. Startups and mid-tier companies are differentiating through AI-powered forecasting engines that predict vaccine utilization trends, enabling proactive stock management and tailored patient outreach.
Cross-industry collaborations-such as alliances between logistics firms and software integrators-are emerging to address cold chain complexities and ensure end-to-end traceability. As competition intensifies, vendors that combine domain expertise in immunization workflows with flexible deployment options and comprehensive support offerings are poised to capture the growing demand for integrated solutions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Vaccine Administration Management System market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Allscripts Healthcare, LLC
- athenahealth, Inc.
- Computer Programs and Systems, Inc.
- eClinicalWorks, LLC
- Epic Systems Corporation
- GE HealthCare Technologies, Inc.
- McKesson Corporation
- Medical Information Technology, Inc.
- NextGen Healthcare, Inc.
Strategic Imperatives and Practical Recommendations to Capitalize on Market Opportunities and Navigate Emerging Challenges in Vaccine Administration Management
To navigate an increasingly complex market, industry leaders should prioritize the adoption of interoperable, cloud-native architectures that support rapid scaling and seamless integration with electronic health record systems. Investing in modular software that can be tailored to specific end-user requirements-ranging from public health departments to community clinics and pharmacies-enables organizations to reduce implementation timelines and enhance user satisfaction. Moreover, establishing partnerships with domestic manufacturing and distribution networks can mitigate supply chain risks introduced by geopolitical and tariff fluctuations.
Strategic emphasis on training and support services is crucial; comprehensive onboarding programs and continuous user education ensure high adoption rates and minimize operational disruptions. Embracing data analytics for real-time inventory monitoring and demand forecasting enhances decision making at every level, from local clinics managing pediatric vaccination schedules to national agencies coordinating mass immunization drives. Finally, a phased deployment approach-beginning with critical modules such as compliance tracking and scheduling, followed by advanced analytics and integrated reporting-enables stakeholders to demonstrate early wins, secure executive buy-in, and build momentum for broader digital transformation initiatives.
Explaining Rigorous MultiStage Research Methodology Leveraging Primary and Secondary Sources Expert Interviews Data Validation and Analytical Frameworks
This research initiative employed a multi-stage methodology combining extensive secondary research with targeted primary engagements to ensure depth, accuracy, and relevance. Secondary sources included regulatory filings, white papers, and publicly available industry reports, providing a foundational understanding of market drivers, technology trends, and tariff impacts. Building on this groundwork, expert interviews with senior executives, IT leaders, and end-user representatives across government agencies, hospitals, pharmacies, and academic institutions enriched the analysis with practical insights and real-world perspectives.
A rigorous data validation framework was applied to reconcile discrepancies between sources, while a bottom-up approach was used to map segmentation landscapes across end-user categories, components, deployment modes, and applications. The research team leveraged both quantitative survey data and qualitative case studies to capture nuanced adoption behaviors and procurement criteria. Throughout the process, periodic reviews by an advisory panel of immunization experts and supply chain specialists ensured that the findings accurately reflect current market realities and emerging opportunities.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Vaccine Administration Management System market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Vaccine Administration Management System Market, by End User
- Vaccine Administration Management System Market, by Component
- Vaccine Administration Management System Market, by Deployment Mode
- Vaccine Administration Management System Market, by Application
- Vaccine Administration Management System Market, by Region
- Vaccine Administration Management System Market, by Group
- Vaccine Administration Management System Market, by Country
- United States Vaccine Administration Management System Market
- China Vaccine Administration Management System Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 2703 ]
Synthesizing Key Findings and Reinforcing the Strategic Importance of Advanced Vaccine Administration Management Systems in Strengthening Public Health Outcomes
The evolution of vaccine administration management systems underscores a broader shift toward data-driven, patient-centric healthcare delivery. By harnessing interoperability standards, cloud-native platforms, and advanced analytics, stakeholders can achieve end-to-end visibility, optimize resource utilization, and elevate patient engagement. The collective insights presented herein illuminate how tariff dynamics, market segmentation, regional nuances, and competitive innovations converge to shape strategic imperatives.
As public health priorities intensify and digital maturity accelerates across regions, the imperative for integrated, scalable, and resilient administration management solutions has never been clearer. Organizations that align their technology investments with the needs of diverse end users-while remaining adaptable to regulatory and economic shifts-will be best positioned to deliver safe, efficient, and equitable vaccination experiences.
Engage With Associate Director of Sales and Marketing for Customized Insights and Market Research to Propel Strategic Decision Making and Business Growth
If you are ready to leverage comprehensive insights and secure a competitive edge, reach out to Ketan Rohom, Associate Director of Sales and Marketing. Drawing on an in-depth understanding of emerging trends, regulatory landscapes, and strategic growth levers, Ketan will guide you toward the specific data, analysis, and customized recommendations your organization requires. Whether you seek focused deep dives into end-user adoption dynamics, detailed evaluations of hardware and software solutions, or tailored regional assessments, engaging directly with Ketan ensures prompt, personalized support. Partnering with his team unlocks early access to executive briefings, exclusive analyst hours, and bespoke consulting workshops designed to accelerate time-to-value. Contact Ketan today to discuss your objectives, explore flexible report packages, and embark on a data-driven journey that will empower your senior leadership and drive measurable outcomes in vaccine administration management.

- How big is the Vaccine Administration Management System Market?
- What is the Vaccine Administration Management System Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




