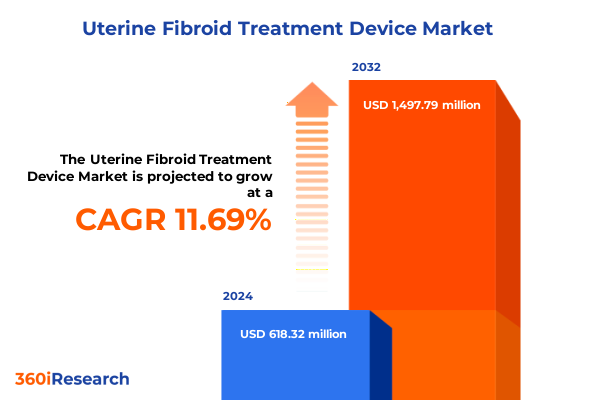
The Uterine Fibroid Treatment Device Market size was estimated at USD 684.20 million in 2025 and expected to reach USD 757.51 million in 2026, at a CAGR of 11.84% to reach USD 1,497.79 million by 2032.
An In-Depth Exploration of the Uterine Fibroid Treatment Device Landscape Driven by Innovation, Patient Needs, and Shifting Healthcare Priorities
The prevalence of uterine fibroids has driven a significant demand for innovative treatment devices, compelling healthcare providers, manufacturers, and policymakers to reevaluate traditional approaches. Driven by a deep understanding of patient experiences and clinical outcomes, industry stakeholders are increasingly prioritizing solutions that minimize invasiveness, enhance precision, and promote faster recovery. Amid rising patient awareness and evolving reimbursement environments, the uterine fibroid treatment device arena is characterized by an accelerating pace of technological advancements and an expanding array of therapeutic options.
As minimally invasive interventions gain traction, the role of device innovation becomes ever more pronounced in shaping care pathways. Next-generation technologies are not only redefining procedure efficacy but are also influencing patient preferences and hospital procurement strategies. With a focus on integrating digital capabilities, data analytics, and personalized care, the landscape is poised for a transformative shift. Consequently, a comprehensive exploration of current trends and strategic imperatives is essential for those seeking to navigate the complexities of this burgeoning market.
How Breakthrough Imaging-Guided and Thermally Mediated Treatment Innovations Are Revolutionizing Uterine Fibroid Care
The uterine fibroid treatment device domain has witnessed a seismic transition from conventional surgical modalities to minimally invasive and image-guided therapies. High-Intensity Focused Ultrasound has emerged as a cornerstone technology, offering patients incision-free procedures that significantly reduce hospital stays and post-operative discomfort. Concurrently, radiofrequency ablation and cryoablation have carved out distinct niches, leveraging thermally mediated tissue ablation to deliver targeted fibroid reduction while preserving uterine integrity. These methods have fundamentally altered clinical protocols, enabling physicians to tailor treatments more precisely to individual uterine fibroid profiles.
Additionally, the integration of advanced imaging modalities-such as real-time ultrasound and magnetic resonance guidance-has elevated the safety and success rates of non-invasive therapies. Interdisciplinary collaboration among radiologists, gynecologists, and biomedical engineers has fostered iterative device refinements and streamlined patient workflows. As a result, the past few years have been marked by accelerated device approvals, an uptick in clinical trial activity, and a growing repository of long-term efficacy data. This critical mass of evidence has, in turn, spurred further investment in next-generation platforms that promise even greater procedural efficiency and patient satisfaction.
Assessing the Far-Reaching Consequences of 2025 US Import Tariffs on Uterine Fibroid Treatment Device Supply Chains and Purchasing Behaviors
The implementation of targeted tariffs on imported medical device components in early 2025 has introduced new complexities into the supply chain for uterine fibroid treatment solutions. Manufacturers reliant on overseas subassemblies have encountered increased production costs, compelling many to reassess sourcing strategies and inventory management. In response, a growing number of original equipment manufacturers have repatriated certain manufacturing operations or forged partnerships with domestic suppliers to mitigate tariff-induced price pressures. These adjustments have yielded both logistical challenges and opportunities to strengthen localized manufacturing capabilities.
Moreover, end users-especially hospitals and ambulatory surgical centers-now face recalibrated procurement dynamics as device pricing structures assimilate additional import duties. Some institutions have deferred capital investments in advanced platforms due to budgetary constraints, while others have prioritized long-term total-cost-of-care calculations over initial acquisition costs. Consequently, the tariff environment is accelerating a strategic pivot toward modular device architectures and vendor agreements that emphasize risk-sharing and flexible pricing models, ultimately redefining value propositions across the market.
Unveiling Critical Market Dynamics Across Product Types, Technological Innovations, Distribution Networks, and End-User Adoption Patterns
By dissecting the market through multiple product type categories, a clear pattern emerges: hysterectomy procedures-particularly laparoscopic and vaginal approaches-continue to command significant clinical adoption, yet high-intensity focused ultrasound and uterine artery embolization are experiencing steeper growth trajectories driven by patient demand for uterine-conserving options. Within the myomectomy segment, hysteroscopic techniques maintain dominance for easily accessible fibroids, while laparoscopic and open methods address more complex cases. Radiofrequency ablation holds a specialized position, appealing to providers seeking a balance between efficacy and minimally invasive delivery.
When evaluating technology segments, the maturity of high-intensity focused ultrasound contrasts with the nascent applications of microwave ablation, which is still undergoing clinical validation. Cryoablation is gaining incremental traction as practitioners seek non-thermal alternatives for patients with specific contraindications. In terms of end users, hospitals remain the primary deployment setting due to their capacity for handling complex cases, but ambulatory surgical centers and specialized fibroid centers are capturing market share by offering streamlined care pathways. Clinics are also broadening services, leveraging referral networks to attract early-stage patients.
Finally, distribution channel dynamics reveal that direct sales models are particularly effective for high-touch devices, while distributors enable broader geographic reach, especially in underserved regions. E-commerce platforms are emerging as a convenient access point for smaller devices and accessories, signaling a digital shift that may influence future purchasing behaviors.
This comprehensive research report categorizes the Uterine Fibroid Treatment Device market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Technology
- End User
- Distribution Channel
Dissecting Regional Market Growth Drivers and Barriers Across the Americas, EMEA, and Asia-Pacific for Uterine Fibroid Devices
Regional analysis highlights distinct growth catalysts in each geographic cluster. In the Americas, reimbursement policies and well-established clinical infrastructure underpin rapid uptake of minimally invasive devices, with specialized fibroid centers emerging as hubs for cutting-edge therapies. Contrastingly, the Europe, Middle East & Africa region exhibits a heterogeneous landscape: Western European markets leverage robust regulatory frameworks to adopt novel modalities swiftly, whereas Middle Eastern and African markets encounter infrastructure and training barriers that temper adoption curves.
Meanwhile, the Asia-Pacific arena stands out for its dual narrative of high patient volume and evolving regulatory environments. China’s investment in high-intensity focused ultrasound platforms has positioned it as both a significant consumer and innovator, while Japan’s stringent device approval processes yield incremental but high-impact advancements in radiofrequency and microwave ablation technologies. India’s cost-sensitive market fosters demand for affordable myomectomy and uterine artery embolization options, driving providers to explore hybrid surgical and device-based protocols. These regional nuances are guiding strategic market entry and localization efforts for global device manufacturers.
This comprehensive research report examines key regions that drive the evolution of the Uterine Fibroid Treatment Device market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Exploring Strategic Collaborations, Technology Advancements, and Portfolio Expansion Efforts Among Leading Uterine Fibroid Device Manufacturers
Key industry participants are leveraging differentiated strategies to enhance their footprint. Legacy medical device corporations with established surgical portfolios are expanding into non-invasive technologies through strategic acquisitions and in-licensing agreements. Simultaneously, specialist firms are investing heavily in the clinical validation of next-generation ultrasound and ablation platforms, often in collaboration with academic medical centers. Several innovative startups have introduced robotic assistance and advanced imaging algorithms, signaling a convergence of automation and precision therapy.
Meanwhile, cross-sector partnerships between device manufacturers and healthcare technology companies are laying the groundwork for integrated digital platforms that connect procedural data to post-treatment outcomes. This ecosystem approach not only enriches real-world evidence but also fosters recurring revenue through software-as-a-service models. As a result, the competitive landscape is increasingly defined by the ability to deliver end-to-end solutions-from preoperative planning and guided intervention to longitudinal patient monitoring.
This comprehensive research report delivers an in-depth overview of the principal market players in the Uterine Fibroid Treatment Device market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbott Laboratories
- Acessa Health Inc.
- AngioDynamics, Inc.
- Boston Scientific Corporation
- Cook Group Incorporated
- CooperSurgical, Inc.
- Gynesonics, Inc.
- Hologic, Inc.
- INSIGHTEC Ltd.
- Karl Storz SE & Co. KG
- Medtronic plc
- Merit Medical Systems, Inc.
- Minerva Surgical, Inc.
- Olympus Corporation
- Profound Medical Corp.
- Richard Wolf GmbH
- SonaCare Medical, LLC
- Varian Medical Systems, Inc.
Strategic Playbook for Device Innovators: R&D Focus, Localization, and Omnichannel Approaches to Accelerate Market Entry and Adoption
Industry leaders should prioritize investment in research and development initiatives that advance non-thermal, image-guided treatment modalities to address evolving patient preferences and minimize procedural risks. Establishing or strengthening domestic manufacturing partnerships can mitigate tariff-related uncertainties, ensuring continuity in critical device supply. Furthermore, cultivating alliances with specialized fibroid centers and key opinion leaders will accelerate clinical adoption and generate high-quality evidence to support value-based contracting.
In addition, adopting omnichannel distribution strategies-integrating direct sales, distributor networks, and digital commerce-will enable broader market penetration and more responsive customer support. Tailoring region-specific go-to-market approaches, with localized training programs and regulatory navigation assistance, can unlock new growth pockets. Finally, embedding data analytics into post-market surveillance and patient follow-up will not only demonstrate long-term efficacy but also reinforce provider and payer confidence in advanced fibroid treatment solutions.
Employing a Robust One-on-One Expert Interview and Multi-Source Validation Methodology to Deliver Comprehensive Market Intelligence
This analysis integrates qualitative and quantitative insights derived from a multi-tiered research framework. Primary data were gathered through in-depth interviews with leading gynecologists, interventional radiologists, and hospital procurement executives across key geographic regions. These conversations provided firsthand perspectives on procedural preferences, adoption barriers, and unmet clinical needs.
Complementing primary insights, secondary research encompassed a comprehensive review of regulatory filings, device approval databases, clinical trial registries, and peer-reviewed literature. Market segmentation was refined through cross-validation against procedural volume statistics and healthcare infrastructure indices. Finally, all findings underwent rigorous validation by subject-matter experts to ensure accuracy and relevance, enabling stakeholders to make informed strategic decisions in the uterine fibroid treatment device landscape.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Uterine Fibroid Treatment Device market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Uterine Fibroid Treatment Device Market, by Product Type
- Uterine Fibroid Treatment Device Market, by Technology
- Uterine Fibroid Treatment Device Market, by End User
- Uterine Fibroid Treatment Device Market, by Distribution Channel
- Uterine Fibroid Treatment Device Market, by Region
- Uterine Fibroid Treatment Device Market, by Group
- Uterine Fibroid Treatment Device Market, by Country
- United States Uterine Fibroid Treatment Device Market
- China Uterine Fibroid Treatment Device Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Concluding Insights Emphasizing the Necessity of Adaptive Strategies, Evidence-Based Innovation, and Regional Tailoring in Fibroid Treatment Device Markets
The uterine fibroid treatment device landscape is undergoing a profound transformation driven by patient-centric innovation, regulatory evolution, and operational imperatives. With non-invasive and minimally invasive modalities gaining prominence, providers and manufacturers alike are compelled to adapt through strategic collaborations, supply chain optimizations, and evidence-generation initiatives. Regional market nuances further underscore the importance of localized strategies and flexible distribution models.
Ultimately, success in this dynamic environment hinges on the ability to anticipate shifting clinical paradigms, respond swiftly to policy changes such as tariff adjustments, and continuously demonstrate value to both providers and payers. Through a concerted focus on cutting-edge technologies, robust data ecosystems, and patient engagement frameworks, industry participants can secure a sustainable competitive advantage and deliver improved outcomes for women affected by uterine fibroids.
Unlock Critical Uterine Fibroid Treatment Device Market Intelligence with Expert Guidance from the Associate Director of Sales & Marketing
To secure comprehensive insights into the nuances of the uterine fibroid treatment device market and gain a competitive edge in this dynamic space, reach out to Ketan Rohom, Associate Director of Sales & Marketing, for an exclusive consultation. By connecting with Ketan, you will receive a tailored overview of key findings, strategic recommendations, and detailed analyses designed to inform critical investment and operational decisions. His expertise will guide you through the full breadth of the report’s in-depth coverage, helping you capitalize on emerging trends and navigate regulatory landscapes effectively. Initiate your journey toward smarter decision-making by contacting Ketan today, and empower your organization with the clarity needed to lead in the ever-evolving uterine fibroid treatment device market.

- How big is the Uterine Fibroid Treatment Device Market?
- What is the Uterine Fibroid Treatment Device Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




