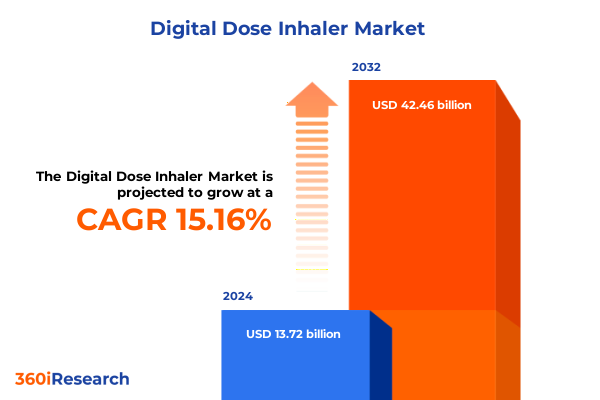
The Digital Dose Inhaler Market size was estimated at USD 15.67 billion in 2025 and expected to reach USD 17.89 billion in 2026, at a CAGR of 15.30% to reach USD 42.46 billion by 2032.
Unveiling the Rise of Digital Dose Inhalers and Their Role in Shaping the Future of Precision Respiratory Care with Connected Technologies
Digital dose inhalers are redefining the nexus of respiratory care and digital health by integrating precise drug delivery with real-time data tracking. This convergence of pharmacotherapy and connected technology has emerged as a pivotal advancement for clinicians and patients alike, fostering enhanced adherence, improved diagnostic insights, and personalized treatment adaptations.
Against the backdrop of escalating chronic respiratory diseases globally, digital dose inhalers are positioned at the vanguard of efforts to optimize therapeutic outcomes. They afford healthcare professionals the ability to monitor inhalation events, assess usage patterns, and adjust dosing regimens remotely, thereby reducing the burden on traditional healthcare settings and promoting proactive patient engagement.
In recent years, robust developments in sensor miniaturization, wireless communication protocols, and cloud-based analytics have catalyzed the transition from conventional inhalers to intelligent devices. As the ecosystem matures, interoperability with electronic health records and patient engagement platforms is becoming a critical focus, underscoring the broader trend toward holistic, technology-enabled healthcare frameworks.
Ultimately, the ascendance of digital dose inhalers signifies a landmark shift in respiratory therapy, transforming management models from reactive to anticipatory care. This introduction sets the stage for a comprehensive exploration of the technological, regulatory, and market dynamics propelling this transformation.
Analyzing the Pivotal Technological and Market Shifts That Are Driving Digital Dose Inhaler Adoption and Redefining Patient Outcomes
The landscape of respiratory therapeutics is experiencing unprecedented transformation driven by converging technological innovations and evolving patient expectations. At the heart of this shift are advanced sensor technologies that capture inhalation force, timing, and environmental factors, empowering clinicians with actionable real-time insights. In parallel, enhanced wireless connectivity standards are facilitating seamless data exchange between devices and care teams, reducing latency and improving the continuity of patient monitoring.
Moreover, the integration of machine learning algorithms is enabling predictive analytics to anticipate exacerbations and tailor interventions before clinical deterioration occurs. This shift from descriptive to predictive models of care is redefining efficacy benchmarks and optimizing resource allocation within healthcare systems. As these digital dose inhaler platforms become more sophisticated, they are also driving novel reimbursement frameworks centered on outcome-based value propositions.
Concurrently, patient-centric design philosophies are reshaping device ergonomics and user interfaces to foster adherence and usability across diverse demographics. By aligning technological capabilities with end-user needs, manufacturers are bridging the gap between innovation and everyday clinical practice. Consequently, the digital dose inhaler market is transitioning from early adoption to mainstream integration, marking a pivotal era in respiratory care evolution.
Assessing How the 2025 United States Tariffs Are Reshaping Digital Dose Inhaler Supply Chains, Pricing Dynamics, and Competitive Landscapes
The implementation of new tariffs on medical devices by the United States in early 2025 has introduced significant variables into the global supply chain of digital dose inhalers. Increased import duties on key components, including microelectronic sensors and wireless modules, have elevated production costs and compelled manufacturers to reassess sourcing strategies. Consequently, device makers are exploring local assembly incentives and diversified vendor partnerships to mitigate tariff-induced margin pressures.
In addition, this tariff regime has influenced pricing negotiations with healthcare providers and payers. As the costs of finished devices ascend, some stakeholders are advocating for revised reimbursement rates to sustain patient access and maintain alignment with value-based care arrangements. Manufacturers are responding by emphasizing total cost of care reduction through improved adherence metrics, demonstrating that higher upfront device costs can be offset by long-term savings in exacerbation management and hospital utilization.
Furthermore, the tariff-induced supply chain recalibrations have accelerated regionalization of production footprints. Strategic investment in North American manufacturing hubs is under consideration to bypass import duties and enhance responsiveness to market demand. While these adjustments introduce transitional complexities, they also present opportunities for elevating domestic capabilities and fostering greater supply chain resilience in the face of future trade fluctuations.
Dissecting the Market Segmentation Landscape to Uncover Critical Patient, Therapeutic, Technological, and Distribution Insights for Digital Inhalers
The digital dose inhaler market is characterized by nuanced patient and therapeutic segments, each demanding tailored device functionalities and engagement strategies. When viewed through the lens of clinical application, asthma management requires solutions adaptable to both adult and pediatric populations, accounting for differential dosing requirements and user dexterity. In contrast, chronic obstructive pulmonary disease demands versatile platforms capable of addressing mild, moderate, and severe disease states, with algorithms calibrated to detect subtle shifts in respiratory patterns across the spectrum of disease progression.
Equally critical is the categorization by drug type, as the inhaler ecosystem must support a diverse portfolio that spans fixed-dose combination therapies, standalone inhaled corticosteroids, long-acting beta agonists, and short-acting beta agonists. Device interfaces must accommodate multi-dose formulations while ensuring accurate dose counters and adherence prompts that align with the pharmacokinetics of each drug class. This complexity underscores the imperative for interoperability between inhaler platforms and medication management software to deliver seamless therapeutic experiences.
Consideration of end-user environments reveals distinct operational demands. Ambulatory care settings and hospital networks require robust integration with institutional IT systems, stringent data security protocols, and scalable device fleets. Conversely, home care scenarios emphasize ease of use, minimal maintenance, and connectivity through ubiquitous consumer devices. Clinics and specialty care centers represent an intermediate domain where hybrid models of in-person training and remote monitoring coexist, necessitating adaptable deployment strategies.
Moreover, technological segmentation delineates the market by connectivity modalities-Bluetooth enabled, near field communication enabled, and radio frequency enabled configurations. Each pathway offers trade-offs in power consumption, data transfer rates, and pairing complexities that influence device lifecycle management. Finally, distribution channels ranging from direct sales engagements and hospital distributors to online and retail pharmacy networks shape market accessibility, inventory dynamics, and end-to-end patient fulfilment pathways. Taken together, these overlapping segmentation dimensions illuminate the multifaceted nature of the digital dose inhaler landscape and the tailored approaches required to address distinct stakeholder requirements.
This comprehensive research report categorizes the Digital Dose Inhaler market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Device Configuration
- Drug Type
- Inhaler Platform
- Application
- End User
- Distribution Channel
Mapping Regional Nuances and Growth Drivers Across the Americas, Europe Middle East & Africa, and Asia-Pacific for Digital Dose Inhaler Expansion
Regional market dynamics for digital dose inhalers are influenced by regulatory frameworks, healthcare infrastructure maturity, and patient care models that vary substantially across geographies. In the Americas, established reimbursement pathways for remote patient monitoring and strong digital health adoption support early deployment of connected inhaler solutions. Collaborative ecosystems involving payers, providers, and technology vendors are forging proof-of-concept pilots that underscore real-world value and pave the way for scaled rollouts.
Across Europe, the Middle East, and Africa, disparate regulatory environments and inconsistent digital infrastructure present both challenges and avenues for innovation. Regions with integrated health systems and centralized electronic health records are more conducive to rapid adoption, while areas with nascent digital health policies may require more rigorous evidence generation and stakeholder education initiatives. Meanwhile, cost-containment imperatives in many European markets drive interest in outcome-based contracting models that incentivize adherence and long-term disease management efficiencies.
In the Asia-Pacific region, expanding mobile connectivity and rising prevalence of asthma and chronic obstructive pulmonary disease create fertile grounds for digital therapeutics. Despite variability in healthcare spending per capita, public–private partnerships and government initiatives are investing in telehealth frameworks to extend care to underserved populations. Localized manufacturing hubs and supportive trade policies further bolster market entry for device manufacturers.
Taken in sum, regional disparities are pronounced, but they also highlight strategic inflection points. Stakeholders can harness lessons from mature markets to tailor entry strategies in emerging regions, aligning device capabilities with unique payer landscapes, distribution mechanisms, and patient engagement paradigms.
This comprehensive research report examines key regions that drive the evolution of the Digital Dose Inhaler market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Illuminating the Strategies, Partnerships, and Innovations of Leading Players Driving Competitive Advantage in the Digital Dose Inhaler Market
Leading companies in the digital dose inhaler arena are distinguished by their multifaceted strategies encompassing product innovation, strategic alliances, and ecosystem integration. Market incumbents have invested heavily in proprietary sensor technologies and data analytics platforms, enabling them to differentiate through granular adherence tracking and predictive health insights. Concurrently, several firms are forging partnerships with pharmaceutical developers to co-develop inhalers that seamlessly integrate with specific therapeutic formulations.
In addition to vertical collaborations, device manufacturers are seeking interoperability with broader digital health ecosystems, integrating their inhaler data streams into population health management solutions and telehealth portals. These alliances enhance the utility of the inhaler platforms by contextualizing usage data within comprehensive patient health records, thereby creating richer decision support pathways for clinicians.
Furthermore, selective acquisitions and venture investments have diversified the portfolios of key players, adding capabilities such as mobile app development, cloud security, and machine learning. By embedding these competencies, companies are accelerating time-to-market for next-generation devices and reinforcing competitive moats against new entrants. The interplay of organic R&D efforts and inorganic growth strategies underscores the dynamic competitive landscape and the imperative for continuous innovation.
This comprehensive research report delivers an in-depth overview of the principal market players in the Digital Dose Inhaler market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- 3M Company
- Adherium Limited
- AptarGroup, Inc.
- AstraZeneca plc
- Boehringer Ingelheim International GmbH
- Cipla Limited
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Limited
- H&T Presspart Manufacturing Ltd.
- Koninklijke Philips N.V.
- Novartis AG
- OPKO Health, Inc.
- ResMed Inc.
- Sensirion AG
- Teva Pharmaceutical Industries Ltd.
Formulating Pragmatic Strategies and Operational Best Practices for Industry Leaders to Capitalize on Opportunities in Digital Dose Inhaler Deployment
Industry leaders seeking to capitalize on the digital dose inhaler opportunity must adopt a holistic approach that spans product development, clinical validation, and stakeholder engagement. First, prioritizing patient-centric design through iterative usability testing will ensure devices are intuitive across age groups and disease severities. Emphasizing seamless connectivity with widely adopted consumer devices and healthcare IT systems will enhance adoption and integration.
Second, forging strategic alliances with pharmaceutical companies and payers is essential to embed digital inhalers within care pathways and reimbursement models. Co-developing outcome-based contracts that link device utilization to adherence metrics and clinical outcomes can unlock shared value propositions and mitigate pricing pressures. Moreover, generating robust real-world evidence through pilot programs and registries will substantiate clinical and economic benefits.
Third, optimizing supply chain resilience through regionalized manufacturing and diversified sourcing will mitigate risks associated with trade policy fluctuations and component shortages. Finally, investing in data security and compliance frameworks will safeguard patient privacy and align with global regulatory mandates, thereby reinforcing trust among healthcare partners.
By executing these integrated strategies, industry leaders can not only surmount current market barriers but also establish sustainable competitive positions in the evolving digital respiratory care ecosystem.
Detailing Robust Research Approaches, Data Collection Frameworks, and Analytical Techniques Underpinning the Digital Dose Inhaler Market Study
This study leverages a multi-tiered research approach combining primary and secondary data collection, triangulated with expert interviews and stakeholder consultations. Initially, a comprehensive literature review was conducted across peer-reviewed journals, clinical trial databases, and industry white papers to map existing technologies, regulatory guidelines, and patient adherence metrics.
Subsequently, in-depth interviews with pulmonologists, respiratory therapists, and digital health specialists provided qualitative insights into clinical workflow integration, patient engagement challenges, and technology adoption drivers. These findings were augmented by surveys of device manufacturers, distributors, and payers to quantify market dynamics and emerging business models.
Quantitative analysis involved the examination of device shipment data, tariff schedules, and procurement records, calibrated to account for regional trade policy impacts and distribution channel performance. Advanced statistical techniques were employed to identify correlations between device usage patterns and clinical outcomes, while scenario modeling assessed the potential implications of tariff adjustments and regulatory shifts.
Finally, the research methodology prioritized data validation through cross-functional peer reviews and alignment with international healthcare frameworks. This rigorous approach underpins the credibility of the findings and ensures that the strategic recommendations are grounded in empirically verified evidence.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Digital Dose Inhaler market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Digital Dose Inhaler Market, by Device Configuration
- Digital Dose Inhaler Market, by Drug Type
- Digital Dose Inhaler Market, by Inhaler Platform
- Digital Dose Inhaler Market, by Application
- Digital Dose Inhaler Market, by End User
- Digital Dose Inhaler Market, by Distribution Channel
- Digital Dose Inhaler Market, by Region
- Digital Dose Inhaler Market, by Group
- Digital Dose Inhaler Market, by Country
- United States Digital Dose Inhaler Market
- China Digital Dose Inhaler Market
- Competitive Landscape
- List of Figures [Total: 18]
- List of Tables [Total: 2067 ]
Synthesizing Core Findings and Strategic Implications from the Digital Dose Inhaler Report to Empower Stakeholders with Actionable Clarity
The comprehensive assessment of the digital dose inhaler landscape reveals a convergence of technological innovation, regulatory evolution, and strategic collaboration that is redefining respiratory care. Key drivers include the maturation of connected device platforms, expanding reimbursement models aligned with value-based care, and intensified focus on patient engagement through data-driven insights.
Regional variances underscore the necessity for tailored market entry strategies, with the Americas leading in early adoption, EMEA demonstrating selective growth influenced by reimbursement frameworks, and Asia-Pacific emerging as a high-potential frontier fueled by telehealth initiatives. Leading companies are differentiating through sensor accuracy, analytics capabilities, and ecosystem partnerships, while emerging players are disrupting through nimble innovation and specialized niche offerings.
Against the backdrop of evolving U.S. trade policies, manufacturers are optimizing supply chain resilience and advocating for reimbursement structures that reflect the long-term clinical and economic benefits of digital therapeutics. Ultimately, stakeholders equipped with a nuanced understanding of segmentation dynamics, region-specific drivers, and competitive strategies will be best positioned to navigate this transformative market.
In synthesis, the digital dose inhaler sector stands at an inflection point where data, devices, and digital care models intersect to deliver unprecedented opportunities for improved patient outcomes and system efficiency.
Engage with Ketan Rohom to Unlock Comprehensive Digital Dose Inhaler Market Insights and Empower Strategic Decisions for Future Growth
To delve deeper into these insights and secure a competitive edge, connect directly with Ketan Rohom, Associate Director of Sales & Marketing, who can facilitate access to the full Digital Dose Inhaler market research report. His expertise will guide your procurement process, ensuring you obtain tailored data and strategic recommendations that align precisely with your organization’s objectives. Engage with him to explore customized packages, gain early access to actionable intelligence, and leverage his industry acumen to translate insights into impactful business decisions. Seize this opportunity to fortify your strategies with authoritative research and propel your initiatives forward.

- How big is the Digital Dose Inhaler Market?
- What is the Digital Dose Inhaler Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




