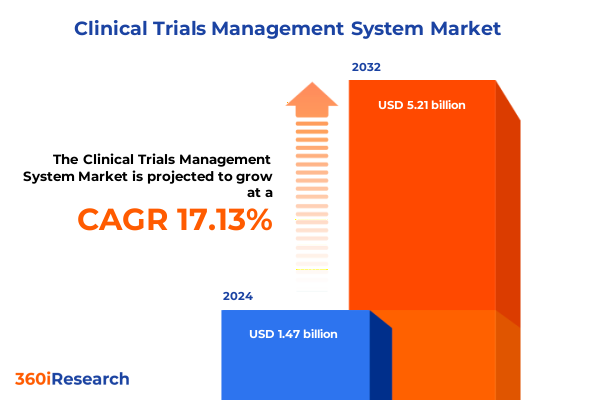
The Clinical Trials Management System Market size was estimated at USD 1.69 billion in 2025 and expected to reach USD 1.96 billion in 2026, at a CAGR of 17.37% to reach USD 5.21 billion by 2032.
Exploring the Critical Role of Innovative Clinical Trials Management Systems in Streamlining Research Operations and Accelerating Therapeutic Advances
Clinical trials management systems play a pivotal role in orchestrating complex research workflows and ensuring data integrity throughout the lifecycle of a study. In an era marked by rapid scientific innovation and heightened regulatory scrutiny, these platforms serve as the backbone for coordinating activities across diverse stakeholders, from investigators and site staff to sponsors and regulatory bodies. By centralizing trial design, monitoring, and data capture, modern systems enhance operational efficiency while safeguarding patient safety and compliance with global standards.
This executive summary distills critical insights across technological disruptions, policy changes, and evolving service models that define the current clinical trials landscape. Drawing upon qualitative research, industry interviews, and regional analyses, it highlights transformative trends, the implications of recent tariff shifts in the United States, and the strategic segmentation frameworks that inform decision-making. Through this synthesis, readers will gain a clear understanding of market dynamics and actionable pathways to optimize trial management workflows, mitigate emerging risks, and accelerate therapeutic development.
Unveiling the Paradigm Shifts Shaping Clinical Trial Ecosystems with Emerging Technologies, Patient-Centric Approaches, and Regulatory Innovations
The clinical trials ecosystem is undergoing a profound transformation driven by digital innovation and a renewed focus on patient centricity. Advances in artificial intelligence and machine learning are automating data cleaning and enhancing predictive analytics, enabling sponsors to forecast enrollment trends and adjust site activation strategies in real time. Concurrently, decentralized trial models leveraging telemedicine, wearable sensors, and remote monitoring are expanding access to underrepresented populations and reducing participant burden.
Regulatory agencies are also adapting to this evolution by offering guidance on adaptive trial designs and real-world evidence integration. As a result, organizations must navigate an increasingly interwoven landscape of technological capabilities and policy frameworks. This shift necessitates cross-functional collaboration among clinical operations, data management, and compliance teams to harness emerging tools effectively while ensuring adherence to Good Clinical Practice standards. Ultimately, the fusion of digital maturity and regulatory flexibility is reshaping how evidence is generated, analyzed, and submitted, setting a new benchmark for trial agility and robustness.
Assessing the Comprehensive Repercussions of Newly Imposed United States Tariffs in 2025 on Clinical Trial Supply Chains and Operational Expenditures
The introduction of new United States tariffs in early 2025 has reverberated across the clinical trial supply chain, influencing the cost and availability of critical trial supplies and equipment. Study sponsors and service providers have encountered increased expenses for imported diagnostic kits, electronic data capture hardware, and cold-chain logistics solutions. In turn, these higher input costs have prompted a strategic reevaluation of procurement practices and vendor relationships to sustain trial budgets without compromising quality or timelines.
To mitigate the impact, organizations are diversifying supplier networks and localizing manufacturing where feasible, while negotiating multi-year contracts to stabilize pricing. Some providers are reallocating resources toward digital and cloud-based offerings that reduce dependency on physical shipments, thereby buffering against tariff volatility. Moreover, trial teams are collaborating more closely with regulatory liaisons to expedite import permits and streamline customs processes. As a result, a resilient supply chain framework is emerging, one that balances cost containment with agility and maintains uninterrupted trial progress despite shifting trade policies.
Deriving Actionable Intelligence from Multi-Dimensional Patient and Service Segmentation to Propel Strategic Decision Making in Clinical Trial Management
Insights from a multi-layered segmentation approach reveal how distinct service lines and therapeutic focuses drive tailored strategies in trial management. Within therapeutic areas such as cardiology, central nervous system disorders, endocrinology, infectious diseases, and oncology, sponsors must align protocol complexity with specialized data capture and monitoring capabilities. For instance, oncology trials often require sophisticated eCRF workflows, whereas central nervous system studies benefit from integrated Randomization and Trial Supply Management functionalities.
The division of trial management services into data management, monitoring, patient recruitment, regulatory submission support, and site management underscores the value of modular deployment. Data management itself encompasses eCRF management, electronic data capture, and supply management modules, enabling sponsors to mix and match functionalities based on study scope. Similarly, site management spans selection, monitoring, and training services, highlighting the necessity of comprehensive site readiness. When differentiating by study type, expanded access, interventional, and observational designs demand varied resource allocations and compliance oversight. End users from academic institutions, contract research organizations, medical device companies, and pharmaceutical biotech firms also leverage these differentiated services to address unique operational imperatives.
Finally, choices between cloud and on-premise deployments influence system scalability and cost structures. Cloud offerings, including hybrid, private, and public clouds, deliver rapid provisioning and cross-site collaboration, while on-premise solutions based on annual subscriptions, licensed software, or perpetual licenses offer greater control over data residency. Together, this segmentation framework empowers stakeholders to craft configurations that align with therapeutic objectives, risk tolerances, and IT policies.
This comprehensive research report categorizes the Clinical Trials Management System market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Therapeutic Area
- Trial Management Service
- Study Type
- Deployment Mode
- End User
Illuminating Regional Variations and Emerging Opportunities Across the Americas, Europe Middle East & Africa, and the Asia-Pacific Clinical Trials Landscape
Regional dynamics are shaping the adoption and evolution of trial management solutions in distinct ways across the Americas, Europe Middle East & Africa, and Asia-Pacific. In the Americas, industry-leading sponsors and service organizations benefit from robust digital infrastructure and comprehensive regulatory guidance, driving rapid uptake of integrated eClinical suites and real-time analytics capabilities. At the same time, localized initiatives to support community-based trial sites are enhancing enrollment diversity and patient engagement.
Over in Europe Middle East & Africa, a multiplicity of regulatory frameworks creates both challenges and opportunities for harmonized trial management. Collaborative efforts through pan-European networks and regulatory convergence programs are helping to streamline cross-border studies, particularly in oncology and rare diseases. Meanwhile, the Gulf region’s investments in research free zones are attracting global sponsors to piloting advanced decentralized trial modalities.
In the Asia-Pacific region, governments and industry leaders are forging public–private partnerships to modernize trial infrastructure, especially in rapidly growing markets like China and India. This focus on capacity building is accelerating the adoption of cloud-native platforms and mobile-enabled patient portals. Yet, the heterogeneous nature of regulatory timelines and data localization requirements demands adaptable deployment strategies, reinforcing the importance of flexible architecture and local expertise.
This comprehensive research report examines key regions that drive the evolution of the Clinical Trials Management System market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting Leading Industry Players and Innovative Collaborations Redefining Competitive Dynamics in Clinical Trials Management Services
The competitive landscape in clinical trials management is defined by a combination of established technology providers and emerging niche players. Market leaders are investing heavily in AI-driven analytics, mobile engagement tools, and interoperable frameworks that seamlessly integrate with electronic health records. Strategic collaborations between global sponsors and specialized vendors are further accelerating the rollout of next-generation platforms that prioritize user experience and regulatory compliance.
Innovative partnerships are also reshaping service delivery models. For example, joint ventures between CROs and cloud platform companies have resulted in turnkey solutions for end-to-end trial orchestration, from patient recruitment to data lock. At the same time, alliances with telehealth providers and digital biomarker specialists are enabling sponsors to conduct remote assessments and expand trial access. This wave of collaboration is complemented by in-house R&D initiatives, where larger organizations are developing proprietary modules to address niche therapeutic requirements and advanced analytics use cases.
As competition intensifies, the ability to deliver modular, scalable, and secure solutions is emerging as a key differentiator. Firms that combine comprehensive global support with agile deployment methodologies are well-positioned to win strategic partnerships and drive operational efficiencies across diverse trial portfolios.
This comprehensive research report delivers an in-depth overview of the principal market players in the Clinical Trials Management System market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- ArisGlobal LLC
- BioClinica, Inc.
- Calyx, Inc.
- Forte Research Systems, Inc.
- ICON plc
- International Business Machines Corporation
- IQVIA Inc.
- Labcorp Drug Development, Inc.
- MasterControl, Inc.
- Medidata Solutions, Inc.
- Medpace, Inc.
- OmniComm Systems, Inc.
- OpenClinica, LLC
- Oracle Corporation
- Parexel International Corporation
- RealTime Software Solutions, Inc.
- Syneos Health, Inc.
- Veeva Systems Inc.
- Worldwide Clinical Trials, Inc.
Formulating Strategic and Measurable Recommendations to Empower Stakeholders in Optimizing Clinical Trials Management and Patient Outcomes
To capitalize on the shifting clinical trials landscape, stakeholders should prioritize the integration of modular digital platforms that support adaptive trial designs and decentralized elements. By deploying scalable data capture and analytics tools, sponsors can streamline site activation and maintain real-time oversight of key performance indicators. In parallel, fostering partnerships with local manufacturing and logistics providers will safeguard supply chain continuity in the face of evolving tariff policies and geopolitical uncertainty.
Patient engagement strategies must also evolve, incorporating remote monitoring technologies and mobile communication channels to enhance retention and data quality. Trial operators should collaborate proactively with regulatory bodies to adopt accelerated review pathways for novel trial formats. Equally important is the cultivation of cross-functional teams that bridge clinical, data, and compliance expertise, ensuring cohesive governance structures and rapid issue resolution.
Finally, organizations should invest in continuous training programs to build digital literacy and change management capabilities among site personnel. By adopting this multifaceted approach, industry leaders can achieve higher operational resilience, optimize resource allocation, and deliver more patient-centric trials that uphold scientific rigor and regulatory standards.
Detailing a Rigorous and Transparent Research Methodology Underpinning Insight Generation in Clinical Trials Management Market Analysis
The research underpinning this executive summary leverages a comprehensive methodology that combines primary and secondary data sources. In-depth interviews with clinical operations executives, trial managers, and regulatory experts provided qualitative insights into emerging challenges and best practices. These perspectives were complemented by a review of regulatory guidelines, industry white papers, and case studies to establish a robust contextual framework.
Quantitative validations were carried out by mapping service line usage patterns and deployment preferences across multiple trial portfolios. Furthermore, a detailed segmentation analysis was performed to highlight variations by therapeutic area, trial service type, study design, end user, and deployment mode. To ensure data integrity, triangulation techniques were applied, cross-referencing findings from different sources and validating them with advisory board feedback from leading industry practitioners.
Through this rigorous, transparent approach, the analysis delivers high-confidence insights designed to support evidence-based decision-making. The methodology’s emphasis on stakeholder engagement and data cross-verification ensures that recommendations are grounded in real-world operational realities and reflect the dynamic nature of the clinical trials ecosystem.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Clinical Trials Management System market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Clinical Trials Management System Market, by Therapeutic Area
- Clinical Trials Management System Market, by Trial Management Service
- Clinical Trials Management System Market, by Study Type
- Clinical Trials Management System Market, by Deployment Mode
- Clinical Trials Management System Market, by End User
- Clinical Trials Management System Market, by Region
- Clinical Trials Management System Market, by Group
- Clinical Trials Management System Market, by Country
- United States Clinical Trials Management System Market
- China Clinical Trials Management System Market
- Competitive Landscape
- List of Figures [Total: 17]
- List of Tables [Total: 1431 ]
Concluding Insights Emphasizing the Strategic Imperative of Advanced Clinical Trials Management Systems for Future Research Excellence
The converging trends of digital innovation, regulatory evolution, and supply chain resilience underscore the strategic imperative of advanced clinical trials management systems. As organizations navigate heightened regulatory expectations and patient-centric demands, the ability to deploy modular, interoperable platforms will define competitive advantage. The segmentation frameworks and regional considerations highlighted in this summary offer a roadmap for aligning technological investments with therapeutic priorities and operational complexities.
Looking ahead, stakeholders must remain vigilant to policy shifts, such as trade tariffs and data localization mandates, while embracing the opportunities presented by decentralized trial designs and AI-driven analytics. By synthesizing insights from diverse sources and fostering collaborative ecosystems, sponsors and service providers can accelerate trial timelines with confidence and maintain the highest standards of data integrity and patient safety.
Ultimately, the strategic deployment of next-generation trial management solutions will not only enhance operational efficiency but also catalyze breakthroughs in therapeutic development, advancing scientific discovery and improving patient outcomes globally.
Engage Directly with Ketan Rohom to Secure Essential Insights and Drive Clinical Trials Management Excellence Through a Customized Market Research Report
Engaging with Ketan Rohom offers a strategic pathway to leverage specialized expertise in clinical trials management. He brings a wealth of experience in aligning sales and marketing strategies with research insights, ensuring that organizations can harness the full breadth of the report’s findings. By collaborating directly, stakeholders will gain access to tailored recommendations and deeper analyses that address unique operational challenges, ranging from regulatory compliance to patient-centric trial design. This engagement facilitates a custom approach to deploying best practices across data management, site operations, and study execution, thus maximizing return on investment.
Readers are encouraged to initiate a conversation with Ketan Rohom to explore customized service packages and priority access to the latest modules of the research report. His role as Associate Director in Sales & Marketing underscores a commitment to delivering high-impact solutions designed to propel trial efficiency and stakeholder collaboration. Taking this step will not only secure comprehensive insights but also position your organization at the forefront of clinical trial innovation, ensuring readiness for evolving industry demands and regulatory landscapes.

- How big is the Clinical Trials Management System Market?
- What is the Clinical Trials Management System Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




