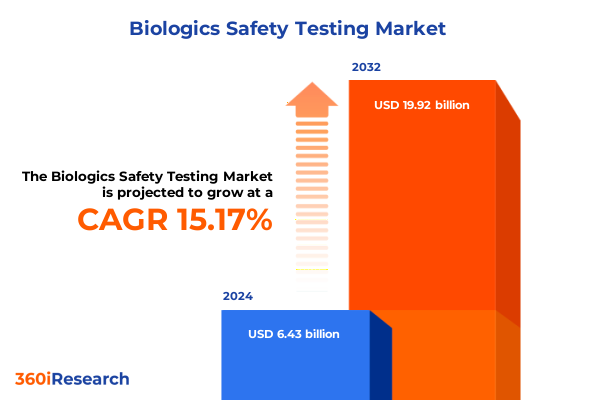
The Biologics Safety Testing Market size was estimated at USD 7.30 billion in 2025 and expected to reach USD 8.29 billion in 2026, at a CAGR of 15.41% to reach USD 19.92 billion by 2032.
Exploring the Evolution of Biologics Safety Testing: Groundbreaking Innovations and Emerging Regulatory Alignments Driving Research Excellence
In response to the unprecedented complexity of modern biologics therapies, safety testing has undergone a remarkable evolution that blends technological ingenuity with regulatory foresight. Novel modalities such as gene-edited cell therapies and multifunctional antibody conjugates have increased the demand for advanced analytical platforms, driving the integration of high-resolution mass spectrometry, next-generation sequencing, and automated cell culture workflows. Moreover, stakeholders are collaborating across public and private sectors to harmonize standards, expedite approvals, and ensure consistent quality benchmarks for emerging products.
As these innovations reshape the biologics safety paradigm, laboratories worldwide are adopting digital data management systems and artificial intelligence–powered analytics to streamline workflows and reduce manual error. Consequently, organizations are better equipped to detect trace impurities, authenticate cell lines, and monitor adventitious agents with unprecedented precision. In addition, the growing emphasis on continuous process verification reflects a strategic shift toward real-time risk mitigation, linking in-process controls directly to end-product release criteria.
Identifying the Major Paradigm Shifts Reshaping Biologics Safety Testing through Technological Integration and Regulatory Harmonization Across the Industry
The biologics safety testing environment has witnessed a series of paradigm shifts that fundamentally alter how laboratories operate and comply with regulatory mandates. Foremost among these is the move toward automation, where robotic liquid handlers and integrated sample tracking systems enhance throughput while preserving data integrity. In parallel, digital twins of production processes enable predictive monitoring, reducing batch failures and supporting proactive quality control.
Another transformative trend is the adoption of risk-based frameworks aligned with ICH Q9 principles, which prioritize critical quality attributes and focus resources on the most significant safety risks. This approach is complemented by global regulatory convergence, as updated guidelines from the FDA, EMA, and PMDA increasingly reflect shared standards for sterility assurance, endotoxin limits, and mycoplasma detection. Coupled with the rise of continuous manufacturing platforms, these shifts are paving the way for streamlined product lifecycles and more efficient test method transfers across geographies.
Analyzing the Ripple Effects of 2025 United States Tariffs on Biologics Safety Testing Operations Supply Chains and Industry Competitiveness
In mid-2025, newly implemented U.S. tariffs have introduced additional costs to imported testing reagents, critical consumables, and certain instrumentation components essential for biologics safety workflows. These measures, aimed at bolstering domestic manufacturing, have generated immediate price pressures, prompting laboratories to re-evaluate procurement strategies and inventory management.
Consequently, many stakeholders are diversifying their supply chains to include regional distributors and domestic producers of reagents & kits, thereby reducing exposure to cross-border duties. Instrumentation providers have responded by localizing key manufacturing steps, negotiating tariff exclusions for high-risk testing platforms, and offering service-based models to offset capital investment. Moreover, the adjustment in trade policy has accelerated the trend of nearshoring, with several contract research organizations and academic centers establishing new testing hubs closer to end-user clusters.
While these adaptations mitigate short-term disruptions, the cumulative impact of tariffs will likely influence long-term strategic planning. Organizations are now balancing cost containment against the imperative of maintaining rigorous safety standards, underscoring the importance of flexible supply agreements and predictive procurement analytics.
Uncovering Critical Segmentation Insights Spanning Product Types Test Modalities Applications and End User Dynamics in Biologics Safety Testing
A nuanced understanding of market dynamics emerges when examining the interplay among instruments, reagents & kits, and services. Advanced analytical instruments form the backbone of high-precision safety testing, while ready-to-use reagents and kits reduce time to result and standardize procedures. Complementing these components, specialized service offerings deliver end-to-end support, enabling laboratories to scale capacity without substantial capital outlay.
Turning to test types, the landscape spans adventitious agent detection, bioburden testing, cell line authentication & characterization, endotoxin testing, mycoplasma testing, residual host cell protein analysis, and sterility testing. Each modality addresses a distinct risk profile, from viral safety to contamination control, and collectively they constitute a comprehensive defense against product failure. In this context, adopting multi-modal platforms that integrate orthogonal assays has become a best practice, enhancing confidence in safety outcomes.
Applications such as gene therapy, monoclonal antibody production, recombinant protein therapeutics, stem cell research, tissue engineering, and vaccine development each demand tailored testing protocols. As these sectors evolve, the relative emphasis on specific test types shifts, creating opportunities for cross-platform innovation. Finally, the ecosystem of academic and research institutes, contract research organizations, and pharmaceutical & biotechnology companies drives demand patterns, with each end user prioritizing speed, regulatory compliance, or scale in alignment with their strategic goals.
This comprehensive research report categorizes the Biologics Safety Testing market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product Type
- Test Type
- Application
- End User
Evaluating Regional Variations and Growth Drivers across the Americas EMEA and Asia Pacific in the Biologics Safety Testing Ecosystem
Geographic variations exert a significant influence on the adoption and implementation of biologics safety testing protocols. In the Americas, stringent regulatory frameworks and a dense concentration of biotech hubs have cultivated an environment rich in collaborative research and early adoption of advanced testing modalities. This region’s robust infrastructure supports innovation in continuous manufacturing and digital quality practices, reinforcing its leadership position.
Across Europe, the Middle East, and Africa, harmonized standards under the European Medicines Agency, UAE regulatory reforms, and South Africa’s growing bio-economy are driving a convergence of best practices. Regulatory initiatives such as the EU’s revised Annex I have intensified focus on contamination control and aseptic processing, prompting regional stakeholders to invest in state-of-the-art endotoxin and sterility testing solutions.
Meanwhile, the Asia-Pacific region is characterized by rapid expansion of domestic biologics manufacturing in markets such as China and India. Governments are incentivizing local production of reagents, kits, and instruments to support nascent therapeutic pipelines. In addition, partnerships between multinational suppliers and regional distributors are facilitating technology transfers, enabling emerging economies to leapfrog traditional quality control models.
This comprehensive research report examines key regions that drive the evolution of the Biologics Safety Testing market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Profiling Leading Industry Stakeholders and Their Strategic Innovations Driving Competitive Advantage in Biologics Safety Testing Market Landscape
Leading industry stakeholders have adopted distinct strategies to maintain competitive advantage. Thermo Fisher Scientific continues to expand its portfolio with integrated platforms that combine mass spectrometry, chromatography, and automated cell assays, reinforcing its position as a one-stop solutions provider. MilliporeSigma has focused on modular reagent kits and digital assay kits, enabling rapid customization and seamless integration into existing workflows.
Danaher’s portfolio companies have emphasized automation, leveraging robotics and artificial intelligence to reduce manual intervention and standardize results. Charles River Laboratories is deepening its expertise in specialized testing services, targeting modalities such as mycoplasma detection and adventitious virus screening through dedicated center-of-excellence facilities. Similarly, global testing networks operated by Eurofins and SGS are capitalizing on localized laboratory footprints to offer rapid turnaround times and regional regulatory support.
Strategic acquisitions and collaborative alliances remain prevalent as companies seek to bolster their service offerings and geographic reach. Investment in digital platforms, combined with expanded service-based models, underscores a broader shift toward end-to-end partnerships rather than transactional interactions.
This comprehensive research report delivers an in-depth overview of the principal market players in the Biologics Safety Testing market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Agilent Technologies, Inc.
- Associates of Cape Cod, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Charles River Laboratories International, Inc.
- Clean Biologics SAS
- Cytiva Europe GmbH
- Danaher Corporation
- Eurofins Scientific SE
- FUJIFILM Corporation
- GenScript Biotech Corporation
- Intertek Group plc
- Laboratory Corporation of America Holdings
- Merck KGaA
- Microcoat Biotechnologie GmbH
- Pace Analytical Services, LLC
- Promega Corporation
- QIAGEN N.V.
- QPS Holdings LLC
- Samsung Biologics Co., Ltd.
- SGS SA
- Syngene International Limited
- Thermo Fisher Scientific Inc.
- Toxikon Corporation
- WuXi AppTec Co., Ltd.
Implementing High Impact Strategies and Best Practices to Enhance Operational Efficiency Compliance and Innovation in Biologics Safety Testing Organizations
Industry leaders can accelerate growth and resilience by adopting a series of targeted initiatives. Prioritizing automation and digitalization not only enhances data integrity but also reduces cycle times and operational costs. Establishing a dedicated regulatory intelligence function ensures proactive alignment with evolving guidelines, from ICH quality protocols to revised GMP annexes.
Diversifying supply chains through strategic partnerships with regional reagent manufacturers and local instrument assemblers mitigates tariff exposure and shortens lead times. Collaborations with contract research organizations and academic centers foster access to specialized expertise and emerging technologies, driving co-innovation in next-generation safety assays.
Embedding quality by design principles within process development and validation frameworks enables real-time risk management, while continuous monitoring systems provide actionable insights to address deviations promptly. Furthermore, investing in workforce training on digital tools and data analytics cultivates a culture of innovation and positions organizations to capitalize on emerging opportunities in quality testing.
Detailing a Robust Research Methodology Combining Primary Expert Insights Secondary Data Analysis and Qualitative Quantitative Validation for Credibility
The analysis underpinning this report is grounded in a rigorous multi-phase research framework. Comprehensive secondary research involved reviewing peer-reviewed journals, regulatory guidance documents, and industry white papers to map current practices and technological trends. This foundational work was complemented by primary research, including in-depth interviews with senior executives, laboratory directors, quality assurance managers, and regulatory affairs specialists.
Qualitative insights were triangulated with quantitative data derived from laboratory adoption rates, equipment deployment statistics, and reagent consumption metrics provided by select commercial partners. Data validation exercises included cross-verification with published case studies, regulatory filings, and technology benchmarks. Throughout the process, successive review cycles with subject matter experts ensured that findings remain credible, unbiased, and aligned with the latest developments in biologics safety testing.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Biologics Safety Testing market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Biologics Safety Testing Market, by Product Type
- Biologics Safety Testing Market, by Test Type
- Biologics Safety Testing Market, by Application
- Biologics Safety Testing Market, by End User
- Biologics Safety Testing Market, by Region
- Biologics Safety Testing Market, by Group
- Biologics Safety Testing Market, by Country
- United States Biologics Safety Testing Market
- China Biologics Safety Testing Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 795 ]
Summarizing Key Takeaways and Strategic Implications to Empower Decision Makers in Leveraging Biologics Safety Testing Innovations for Sustainable Advancement
In an environment defined by rapid technological innovation and shifting regulatory landscapes, biologics safety testing stands at a pivotal juncture. Advanced analytical platforms, risk-based quality frameworks, and automation are collectively raising the bar for safety assurance. Simultaneously, new trade policies and tariffs have prompted organizations to revisit supply chain strategies, highlighting the need for agility and resilience.
Segmentation analysis reveals diverse requirements across instruments, reagents & kits, services, test types, applications, and end users, underscoring the importance of tailored solutions. Regional dynamics in the Americas, EMEA, and Asia-Pacific each present unique opportunities for market entry and expansion, while key industry players are setting new benchmarks through strategic investments and partnerships.
Ultimately, decision makers must harmonize innovation, regulatory compliance, and operational efficiency to navigate this complex landscape. By leveraging the insights and recommendations provided herein, organizations can strengthen their competitive positioning and ensure the highest standards of safety in biologics development and manufacturing.
Unlock Comprehensive Insights and Gain a Competitive Edge by Securing Your Exclusive Biologics Safety Testing Market Research Report with Expert Guidance
We invite you to harness this wealth of insights by securing the exclusive Biologics Safety Testing market research report today. Partnering with Ketan Rohom, an Associate Director specializing in Sales and Marketing, you will receive personalized guidance to navigate this complex landscape and capitalize on emerging opportunities. Engage for a tailored briefing that aligns with your strategic priorities and gain competitive advantage through actionable intelligence. Reach out now to transform your decision-making and drive long-term success by accessing comprehensive data, expert analysis, and targeted recommendations.

- How big is the Biologics Safety Testing Market?
- What is the Biologics Safety Testing Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




