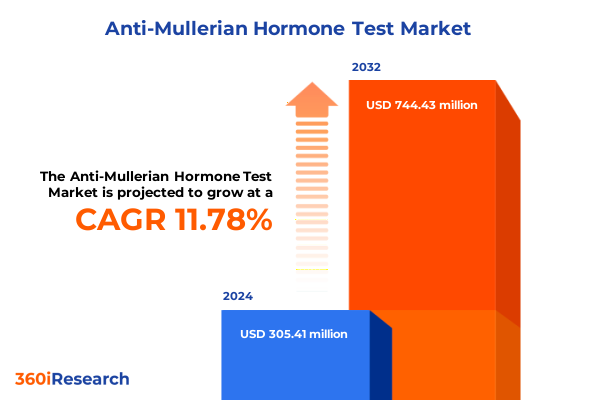
The Anti-Mullerian Hormone Test Market size was estimated at USD 340.21 million in 2025 and expected to reach USD 381.30 million in 2026, at a CAGR of 11.83% to reach USD 744.42 million by 2032.
Exploring the Emerging Role of Anti-Mullerian Hormone Testing as a Cornerstone of Personalized Fertility Assessment and Reproductive Healthcare
Anti-Mullerian Hormone testing has emerged as a cornerstone in the field of reproductive health, offering an objective biomarker for ovarian reserve that transcends traditional subjective assessments. By quantifying ovarian follicle potential with precision, this assay has transformed the landscape of fertility evaluation and family planning. Clinicians now leverage AMH levels to personalize treatment protocols, optimize stimulation regimens in assisted reproductive technologies, and counsel patients with enhanced confidence, thereby improving both clinical outcomes and patient experience.
As the tide shifts toward value-based healthcare, the role of AMH testing continues to expand, driven by its predictive strength and adaptability across care settings. From central laboratories adopting high-throughput platforms to emerging point-of-care environments, the breadth of applications underscores a transition toward more decentralized, patient-centered diagnostics. This section sets the stage for a deeper exploration of technological advancements, market forces, and strategic considerations defining this dynamic diagnostic domain.
Tracing the Transformative Shifts in Diagnostic Innovations and Clinical Practices That Are Redefining the Anti-Mullerian Hormone Testing Landscape
Over the past decade, diagnostic innovations have fundamentally altered the trajectory of AMH testing by integrating automation, digital connectivity, and data analytics. The advent of high-precision immunoassays on benchtop analyzers has enabled laboratories to achieve reproducibility at scale while portable devices are empowering clinicians to bring critical ovarian reserve data directly to the patient’s bedside. Concurrently, advances in assay sensitivity and specificity have refined detection thresholds, thereby facilitating earlier intervention and more nuanced patient stratification.
Regulatory harmonization across major markets and the establishment of standardized performance criteria have accelerated adoption, while manufacturers have collaborated closely with clinical experts to streamline workflows and reduce turnaround times. In parallel, self-check formats are gaining traction among health-aware consumers seeking privacy and convenience, heralding a new era of patient empowerment. Together, these shifts underscore a broader transformation toward accessible, precise, and patient-centric reproductive diagnostics.
Assessing the Cumulative Impact of Evolving United States Tariff Measures on Supply Chain Dynamics and Cost Structures within the AMH Testing Industry
Recent policy actions by the United States government have introduced significant tariff adjustments that reverberate across the supply chains supporting AMH testing. Section 301 tariffs on semiconductor imports rose to 50 percent at the start of 2025, directly impacting the cost base for portable analyzers that incorporate microprocessor technology. Meanwhile, ongoing probes into pharmaceutical ingredients signal potential levies on critical reagents, creating headwinds for both customized assays and standard kits. These measures, cumulatively, have heightened cost pressures and underscored the need for strategic sourcing solutions.
At the same time, protective duties on steel and aluminum indirectly affect infrastructure components used in benchtop devices, while the reinstatement of certain Trump-era tariffs on medical products such as gloves and masks serves as a reminder of the dynamic nature of trade policy. Manufacturers and distributors face the challenge of mitigating margin erosion while preserving supply continuity. In response, many are exploring nearshoring options, establishing regional distribution hubs, and securing long-term supplier agreements to buffer against future levies.
Transitioning from reactive cost-containment to proactive supply-chain resilience, stakeholders are increasingly investing in scenario planning and real-time trade compliance monitoring. By aligning procurement strategies with evolving tariff schedules, AMH testing providers can safeguard profitability and maintain uninterrupted access to critical instruments and reagents.
Unveiling Strategic Segmentation Insights That Illuminate Product, Component, Application, and End User Dynamics Shaping the AMH Testing Ecosystem
A close examination of market segmentation reveals converging dynamics that shape product development and commercialization strategies across the AMH testing ecosystem. Both point-of-care testing systems and self-check kits are capturing investor interest as they cater to distinct yet complementary use cases. While traditional laboratory-based platforms continue to deliver benchmark accuracy, the rise of decentralized formats speaks to an increasing demand for rapid results and patient autonomy.
Underpinning these product categories is a component ecosystem that bifurcates into instruments and reagents. Within the instruments domain, benchtop analyzers offer high throughput for centralized laboratories, while portable devices support decentralized testing in clinics and remote settings. Complementing these are reagents and kits differentiated into customized assays, which enable tailored protocols for specialized research or clinical trials, and standard assay kits designed for broader diagnostic applications.
When viewed through the lens of clinical applications, AMH testing’s utility spans ovarian reserve assessment, where it serves as a critical indicator of fertility potential, to the diagnosis of polycystic ovary syndrome, providing supportive evidence for endocrine disorder management. Further, its predictive value in premature ovarian failure scenarios positions it as an indispensable tool for early intervention. End-user adoption patterns reinforce these trends, with diagnostic laboratories, fertility clinics, hospitals, and research institutes each demonstrating unique priorities-ranging from high-volume processing to specialized protocol development and academic exploration.
This comprehensive research report categorizes the Anti-Mullerian Hormone Test market into clearly defined segments, providing a detailed analysis of emerging trends and precise revenue forecasts to support strategic decision-making.
- Product
- Component
- Applications
- End User
Examining the Key Regional Dynamics and Market Drivers across the Americas, Europe Middle East Africa, and Asia Pacific in Anti-Mullerian Hormone Testing
Regional dynamics play a pivotal role in shaping the adoption and evolution of AMH testing technologies. In the Americas, especially within the United States and Canada, well-established reimbursement frameworks and a supportive regulatory environment have enabled rapid integration of both centralized instruments and point-of-care platforms. Collaboration between industry stakeholders and leading fertility clinics has fostered a nuanced understanding of patient needs and driven continuous innovation.
In Europe, Middle East, and Africa, a diverse tapestry of healthcare systems presents both opportunities and challenges. Regulatory alignment under the European In Vitro Diagnostic Regulation has elevated quality standards, prompting manufacturers to align product portfolios accordingly. Meanwhile, in select Middle Eastern markets, government investments in fertility infrastructure are creating centers of excellence, and in parts of Africa, partnerships with research institutes are catalyzing localized assay development to meet regional healthcare priorities.
Asia-Pacific markets exhibit robust growth potential underpinned by rising fertility awareness, increasing disposable incomes, and expanding healthcare access. China and India, in particular, are witnessing a surge in private clinic networks and telehealth initiatives that leverage AMH testing for personalized care pathways. Across Australia and Southeast Asia, strategic alliances between global diagnostic leaders and local distributors are facilitating technology transfer and ensuring compliance with regional regulatory frameworks.
This comprehensive research report examines key regions that drive the evolution of the Anti-Mullerian Hormone Test market, offering deep insights into regional trends, growth factors, and industry developments that are influencing market performance.
- Americas
- Europe, Middle East & Africa
- Asia-Pacific
Highlighting the Leading Companies and Strategic Collaborations Driving Innovation Product Development and Competitive Advantage in the AMH Testing Sector
Leading companies in the AMH testing space are driving value through a combination of technological innovation, strategic partnerships, and targeted market expansion. Global diagnostics leaders continue to refine assay platforms, integrating advanced optics and microfluidics to enhance throughput and analytical precision. Concurrently, specialized firms are differentiating offerings by developing bespoke reagents and kits that address niche clinical and research requirements.
Strategic collaborations between instrument manufacturers and reagent developers have emerged as a consistent theme, enabling co-development initiatives that streamline workflows and reduce time to market. In parallel, alliances with academic and clinical research centers are accelerating the translation of novel biomarkers and assay formats into validated diagnostic solutions. Beyond core product development, key players are leveraging digital health platforms to enable remote monitoring and data analytics, further extending the value proposition of AMH testing to endocrinologists, gynecologists, and primary care physicians.
In pursuit of global footprint expansion, many companies are forging distribution agreements across emerging markets, while others are establishing localized manufacturing facilities to address regulatory requirements and reduce lead times. By maintaining a balanced portfolio that spans from high-volume laboratory networks to decentralized point-of-care and self-test modalities, these organizations are positioning themselves to capitalize on the full spectrum of market growth opportunities.
This comprehensive research report delivers an in-depth overview of the principal market players in the Anti-Mullerian Hormone Test market, evaluating their market share, strategic initiatives, and competitive positioning to illuminate the factors shaping the competitive landscape.
- Abbexa Limited
- Ansh Labs LLC
- Athenese-Dx
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- bioMérieux S.A.
- BioVendor – Laboratorni Medicina A.S.
- Chengdu VACURE Biotechnology Co., Ltd.
- CTK Biotech, Inc.
- Cusabio Technology LLC
- Danaher Corporation
- Eagle Biosciences, Inc.
- Elabscience Bionovation Inc.
- Epitope Diagnostics, Inc.
- F. Hoffmann-La Roche Ltd.
- Fujirebio Europe N.V. by H.U. Group Holdings, Inc.
- Goldsite Diagnostics Inc.
- Healthy Human Labs Limited (Vitall)
- Kamiya Biomedical Company
- LifeSpan BioSciences, Inc.
- London Gynaecology Limited
- Meridian Bioscience, Inc.
- Monobind Inc.
- Siemens Healthineers AG
- Thermo Fisher Scientific Inc.
- Tosoh Corporation
- Vitrosens Biotechnology Co., Ltd.
Strategic Actionable Recommendations for Industry Leaders to Enhance Innovation Expand Market Reach and Strengthen Value Chains in the AMH Testing Market
To navigate the evolving AMH testing landscape effectively, industry leaders should adopt a multi-pronged strategic approach. First, investing in modular assay platforms that can be adapted for both centralized and decentralized settings will enable rapid response to shifting clinical workflows and enhance patient access. Simultaneously, expanding self-test and point-of-care offerings through partnerships with telehealth and digital health providers can accelerate consumer adoption and generate new revenue streams.
Second, fostering supplier diversification for both instruments and reagents is critical to mitigating exposure to tariff fluctuations and geopolitical uncertainties. Establishing regional distribution hubs and pursuing manufacturing localization in key markets will not only reduce logistical risks but also facilitate compliance with import regulations and reduce time to market.
Finally, cultivating collaborative relationships with regulatory bodies, professional societies, and patient advocacy groups will reinforce credibility, support consensus on performance standards, and drive broader acceptance of AMH testing protocols. By prioritizing data interoperability and investing in real-world evidence initiatives, stakeholders can demonstrate clinical utility and secure favorable reimbursement policies.
Detailing the Rigorous Research Methodology Employed to Deliver Comprehensive Accurate and Insightful Analysis of the Anti-Mullerian Hormone Testing Market
This analysis is grounded in a rigorous, multi-tiered research methodology designed to ensure comprehensive coverage and analytical depth. Secondary research encompassed a review of peer-reviewed journals, regulatory filings, patent databases, and corporate disclosures to capture the latest technological advancements, policy developments, and strategic initiatives within the AMH testing domain.
Primary research included structured interviews with key opinion leaders, laboratory directors, and clinician end users, as well as surveys administered to diagnostic laboratories, fertility clinics, and research institutes. These engagements provided firsthand perspectives on assay performance requirements, operational challenges, and emerging application needs.
Quantitative data was validated through triangulation across multiple sources, including trade associations, clinical trial registries, and proprietary device utilization metrics. A scenario-based sensitivity analysis was conducted to assess the potential impacts of tariff fluctuations and regulatory changes on supply chain resilience and cost structures. Findings were subjected to multiple rounds of internal peer review to confirm consistency, accuracy, and strategic relevance.
This section provides a structured overview of the report, outlining key chapters and topics covered for easy reference in our Anti-Mullerian Hormone Test market comprehensive research report.
- Preface
- Research Methodology
- Executive Summary
- Market Overview
- Market Insights
- Cumulative Impact of United States Tariffs 2025
- Cumulative Impact of Artificial Intelligence 2025
- Anti-Mullerian Hormone Test Market, by Product
- Anti-Mullerian Hormone Test Market, by Component
- Anti-Mullerian Hormone Test Market, by Applications
- Anti-Mullerian Hormone Test Market, by End User
- Anti-Mullerian Hormone Test Market, by Region
- Anti-Mullerian Hormone Test Market, by Group
- Anti-Mullerian Hormone Test Market, by Country
- United States Anti-Mullerian Hormone Test Market
- China Anti-Mullerian Hormone Test Market
- Competitive Landscape
- List of Figures [Total: 16]
- List of Tables [Total: 1113 ]
Drawing Conclusions on the Strategic Implications and Future Trajectory of Anti-Mullerian Hormone Testing for Healthcare Providers and Industry Stakeholders
In conclusion, the AMH testing sector stands at a pivotal juncture, defined by rapid technological innovation, evolving trade policies, and diverse market demands. Point-of-care platforms and self-test kits are reshaping access paradigms, while high-precision benchtop analyzers continue to set benchmarks for centralized laboratories. Component segmentation highlights the interplay between instrument manufacturers and reagent developers, each driving enhancements in assay performance and workflow efficiency.
Regional insights underscore the importance of tailored strategies, as markets in the Americas, EMEA, and Asia-Pacific navigate regulatory complexities and infrastructure disparities. Leading companies are capitalizing on partnerships and digital solutions to maintain competitive advantage, while actionable recommendations point toward modular product development, supply chain diversification, and stakeholder engagement as critical paths to sustainable growth.
As clinical evidence accumulates and healthcare delivery models evolve, stakeholders who proactively integrate these insights will be best positioned to deliver patient-centered solutions, optimize operational excellence, and secure leadership in the expanding AMH testing landscape.
Take Action to Leverage Comprehensive Market Intelligence for AMH Testing by Engaging with Ketan Rohom Associate Director of Sales and Marketing Today
For organizations seeking to gain a competitive edge in the Anti-Mullerian Hormone testing domain, engaging directly with Ketan Rohom, Associate Director of Sales and Marketing, provides a streamlined path to accessing the full breadth of our comprehensive market intelligence report. His in-depth understanding of both scientific developments and commercial imperatives ensures that stakeholders receive tailored insights and strategic support geared toward informed decision-making and accelerated business growth.
To secure your copy of the market research report and initiate a conversation about customized applications or partnership opportunities, reach out to Ketan Rohom today. This report serves as a critical resource for senior executives, R&D leaders, and business development teams aiming to navigate market complexities, optimize product portfolios, and capitalize on emerging growth avenues in the AMH testing landscape.

- How big is the Anti-Mullerian Hormone Test Market?
- What is the Anti-Mullerian Hormone Test Market growth?
- When do I get the report?
- In what format does this report get delivered to me?
- How long has 360iResearch been around?
- What if I have a question about your reports?
- Can I share this report with my team?
- Can I use your research in my presentation?




